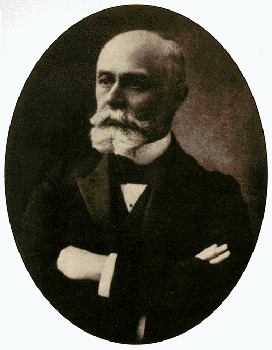Antoine-Henri Becquerel l852 - 1908
http://www.accessexcellence.org/AE/AEC/CC/historical_background.html
 |
Henri Becquerel was born into a family of scientists. His grandfather
had made important contributions in the field of electrochemistry while
his father had investigated the phenomena of fluorescence and phosphorescence.
Becquerel not only inherited their interest in science, he also inherited
the minerals and compounds studied by his father.And so, upon learning
how Wilhelm Roentgen discovered X rays from the fluorescence they produced,
Becquerel had a ready source of fluorescent materials with which to pursue
his own investigations of these mysterious rays.
The material Becquerel chose to work with was potassium uranyl sulfate,
K2UO2(SO4)2, which he exposed
to sunlight and placed on photographic plates wrapped in black paper. When
developed, the plates revealed an image of the uranium crystals. Becquerel
concluded "that the phosphorescent substance in question emits radiation
which penetrates paper opaque to light." Initially he believed that the
sun's energy was being absorbed by the uranium which then emitted X rays.
Further investigation, on the 26th and 27th of February, was delayed
because the skies over Paris were overcast and the uranium-covered plates
Becquerel intended to expose to the sun were returned to a drawer. On the
first of March, he developed the photographic plates expecting only faint
images to appear. To his surprise, the images were clear and strong. This
meant that the uranium emitted radiation without an external source of
energy such as the sun. Becquerel had discovered radioactivity, the spontaneous
emission of radiation by a material.
Later, Becquerel demonstrated that the radiation emitted by uranium
shared certain characteristics with X rays but, unlike X rays, could be
deflected by a magnetic field and therefore must consist of charged particles.
For his discovery of radioactivity, Becquerel was awarded the 1903 Nobel
Prize for physics. |
References:
"The Discovery of Radioactivity and Transmutation"
, edited and with commentary by Alfred Romer, Dover Publications 1964 -
This is a collection of scientific papers from the period 1896 - 1905.
"The Discovery of Radioactivity" , Lawrence
Badash, in Physics Today
February, 1996.
Becquerel, a member of the French Academy of Sciences,
heard Henri Poincare' describe the results of Roentgen's x-ray work at
a regular weekly meeting of the Academy on January 20, 1896. Poincare'
mused on the possibility that the x-rays emanated from the fluorescing
end of the glass vacuum tube where the cathode rays struck. Becquerel's
interest was immediately drawn to this possibility. He, along with his
father, were and had been leading authorities on luminescence. In particular,
he had studied the phosphorescent properties of some uranium compounds.
Becquerel was also quite skilled in photography.
Definitions - Luminescence is the emission of visible
radiation from bodies. There are two types:
fluorescence - stimulation of light emission by an
energy source which disappears when the energy source is removed.
phosphorescence - persistent emission of light from
a body that had been subjected to an external energy source. This emission
continues after the source is removed.
Becquerel's father, Edmond, had shown that some uranium
compounds were phosporesecent, for example, the uranic series, whereas
the uranous series was not. Becquerel's hypothesis was that a luminescing
body also emitted the highly penetrating x-rays of Roentgen. He tested
this hypothesis over a period of about five months in Winter and Spring
of 1896.
"On the Radiation Emitted in
Phosphorescence" , H. Becquerel in
Compte rendus de l'Academie
des Science, Paris, 1896 122: 420-421
(24 February)
He reports that a photographic plate is wrapped in
two sheets of very black paper so that a whole day's exposure to the sunlight
does not fog the plate. If a plate of phosphorescent substance is laid
on the plate and the whole is exposed to the sunlight for several hours
then the plate after being developed shows the outline of the phosphorescent
substance. The image of a coin or thin sheet of metal can also be produced
if the coin or metal is interposed between the phosphorescent substance
and the plate.
"On the Invisible Radiations
Emitted by Phosphorescent Substances"
Compte rendus de l'Academie
des Science, Paris, 1896 122: 501-503
(2 March)
He continues with his hypothesis
that phosphorescent substances emit invisible radiations. For these experiments
he uses a sulfate of potassium and uranium [ K(UO)SO4
+ H2O ] which is brilliantly luminescent and has a very short
persistence of luminescence, 1/100 sec, as shown by his father. As before
he had laid the whole arrangement of covered photographic plate and phosphorescent
minerals in bright sunlight for several hours and found exposure of the
plates to have occurred.
quote from his paper
" I shall particularly insist
on the following fact, which appears to me very important and quite outside
the range of phenomena one might expect to observe. The same crystalline
lamellas ... shielded from excitation by incident radiation and kept in
darkness , still produce the same photographic images."
He came upon this discovery because
there were several days that were overcast and he could not expose his
minerals and plates to the sunlight. He had stored them inside a light
tight drawer, assembled as he would want them for an exposure to sunlight.
It is remarkable that he developed the plates even after they had been
in the dark because he had no reason, based on his hypothesis, to expect
the plates to have been fogged. The images he saw in the developed plates
were as strong as those he saw in the plates with minerals exposed to sunlight.
"A hypothesis which presents
itself rather naturally to mind would be to suppose that these radiations,
whose effects possess a strong analogy with the effects produced
by the radiations studied by Lenard and Roentgen, might be invisible radiations
emitted by phophorescence, whose duration of persistence might be infinitely
greater than that of luminous radiation emitted by these substances."
" On New Properties of the
Invisible Radiation Emitted by Various Phosphorescent Bodies" Compte
rendus de l'Academie des Science, Paris, 1896 122: 501-503 (9 March)
Becquerel announces that the
penetrating rays, like x-rays, have the ability to discharge electrified
bodies. He also believes that the rays could be reflected and refracted
like ordinary light. He had also kept some of his phophorescent crystals
in the dark for 160 hours without any loss of their power to produce the
penetrating rays. He also has narrowed down the list of substances that
can produce rays to compounds of uranium. However, he also reported that
two specimens of calcium sulfide crystals also produced penetrating rays.
" On the Invisible radiations
Emitted by the Salts of Uranium"
Compte rendus de l'Academie
des Science, Paris, 1896 122: 501-503
(23 March)
He reports that he has continued
the use of the electroscope to study the penetrating rays. He compared
the absorption characteristics of the penetrating rays from the "phophorescent"
substances and from the x-rays from a Crookes tube. He also discovered
that the non-luminous compounds of uranium also emit the penetrating rays.
" ... although the salts of
the sequioxide of uranium are very fluorescent, we know that the green
uranous salts ... are neither phophorescent nor fluorescent ... uranous
sulfate behaves like uranic sulfate, and emits invisible radiations which
are equally intense. "
Becquerel also destroyed
the luminscent property of a sample of uranium nitrate. His belief that
the radiation emitted from the uranium compounds was due to energy stored
in the compound led him to expect that destroying the compound would also
destroy the penetrating radiation.
" I shall report still another
interesting experiment. It is known that uranium nitrate ceases to be either
phophorescent or fluorescent when in solution or melted in its water of
crystallization. I then took a crsytal of this salt and, after having placed
it in a little tube closed by a thin plate of glass, I warmed it in darkness,
managing to avoid even the radiations from the alcohol lamp that heated
it. The salt melted, I let it crytallize in darkness and then I placed
it on a photographic plate wrapped in black paper, protecting the salt
always from the action of light. One might have expected to observe no
effect, since all luminous excitation had been avoided from the moment
when the substance ceased to be phosphorescent. Nevertheless the impression
was as strong as for salts exposed to the light..."
"Emission of New Radiations
by Metallic Uranium"
Compte rendus de l'Academie
des Science, Paris, 1896 122: 501-503
(18 May)
By May Becquerel was convinced
that the only requirement to produce the penetrating rays was the presence
of uranium itself. The compounds, luminescent or not, were not the causes
of the emissions. He now began tests with metallic uranium.
" ... I have been led to think
that the effect was due to the presence in these salts of the element uranium,
and that the metal would give more intense effects than its compounds would."
He performed tests using the
photographic technique and the electroscope. At this time there was really
no other language to describe the phenomenon, other than as a type of phosphorescence.
Nevertheless, the energy source for this phophorescence was not identifiable.
" Although I am continuing the
study of these new phenomena, I thought it was not without interest to
point out the emission produced by uranium, which, I believe, is the first
example of a metal exhibiting a phenomenon of the type of an invisible
phophorescence."
Becquerel produced more papers
on uranium's penetrating rays, but there was really nothing much else that
happened until Marie Curie decided to pursue Becquerel's rays as a thesis
topic. Many more scientists were interested in the properties and applications
of Roentgen's x-rays.
In 1903 Becquerel shared the
Nobel prize with Marie and Pierre Curie for the discovery of radioactivity.