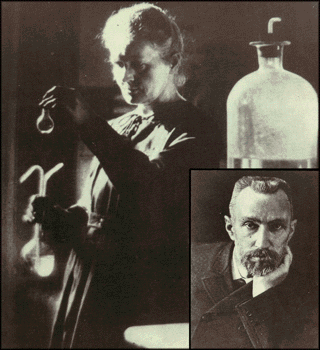Pierre Curie (1859-1906)
Marie Curie (1867-1934)
source: http://www.accessexcellence.org/AE/AEC/CC/historical_background.html
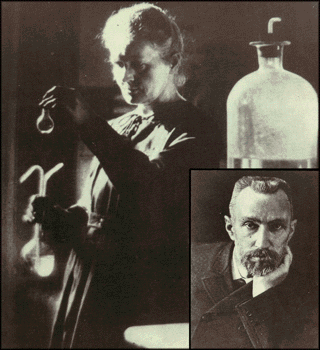
Marie Curie ca. 1920. Inset: Pierre Curie (Marie's favorite picture
of her husband).

The quartz balance, devised by Pierre
Curie and his brother Jacques, was
essential for Marie's work. (Photo ACJC
|
By the time he met Marie Sklodowska, Pierre Curie had already
established an impressive reputation. In 1880, he and his brother Jacques
had discovered piezoelectricity whereby physical pressure applied to a
crystal resulted in the creation of an electric potential. He also had
made important investigations into the phenomenon of magnetism including
the identification of a temperature, the curie point, above which a material's
magnetic properties disappear. However, shortly after his marriage to Marie
in 1895, Pierre subjugated his research to her interests.
Together, they began investigating the phenomenon of radioactivity
recently discovered in uranium ore. Although the phenomenon was discovered
by Henri Becquerel, the term radioactivity was coined by Marie. After chemical
extraction of uranium from the ore, Marie noted the residual material to
be more "active" than the pure uranium. She concluded that the ore contained,
in addition to uranium, new elements that were also radioactive. This led
to their discoveries of the elements of polonium and radium, but it took
four more years of processing tons of ore under oppressive conditions to
isolate enough of each element to determine its chemical properties.
For their work on radioactivity, the Curies were awarded the 1903 Nobel
Prize in physics. Tragically, Pierre was killed three years later in an
accident while crossing a street in a rainstorm. Pierre's teaching position
at the Sorbonne was given to Marie. Never before had a woman taught there
in its 650 year history! Her first lecture began with the very sentence
her husband had used to finish his last. In his honor, the 1910 Radiology
Congress chose the curie as the basic unit of radioactivity: the quantity
of radon in equilibrium with one gram of radium (current definition: 1
Ci = 3.7x1010 dps). A year later, Marie was awarded the Nobel Prize in
chemistry for her discoveries of radium and polonium, thus becoming the
first person to receive two Nobel Prizes. For the remainder of her life
she tirelessly investigated and promoted the use if radium as a treatment
for cancer. Marie Curie died July 4, 1934, overtaken by pernicious anemia
no doubt caused by years of overwork and radiation exposure.
|
References:
"Inward Bound", by Abraham Pais, 1986, Clarendon Press
"Marie Curie - A Life", by Susan Quinn, 1995, Simon
and Schuster
"Madame Curie - A Biography by Eve Curie", Eve Curie,
1938, Doubleday
In December of 1897 Marie Curie began obtaining data
for her doctoral dissertation by investigations into the rays emitted by
uranium. Her "laboratory" was a storage area in the "School of Physics
and Chemistry" where Pierre taught. M. Curie wanted to make a quantitative
study of the "uranic" rays. This seemed to be best done by making electrical
measurements since Becquerel and others after him had shown that the rays
could ionize air. She and Pierre were able to develop a measurement
technique using the precision piezoelectric quartz balance and electrometer
that Pierre had invented along with his brother Jacques Curie. (The Curie
brothers had discovered piezo electricity.)
Her technique involved the use of an ioniztion chamber,
which basically consisted of parallel conducting plates. Ionized air between
the plates provides a conduction path and the current, I, can be measured.
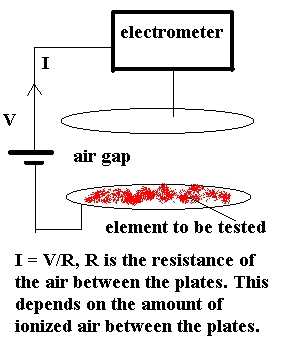
In Curie's case the plates were 8 cm. in diameter
and separated by 3cm. A voltage V, of about 100V was used. Currents, I,
were typically in the range of picoamperes ( 10-12A).
On April 12, 1898, Marie Curie's first paper was presented
at the Academy of Sciences (Paris). There were three important conclusions:
1) She discovered a new active substance, thorium.
This is actually an independent discovery of the same result that G. C.
Schmidt of Erlangen Germany had announced in February of 1898. She had
analyzed a large number of other substances and found no activity. The
discovery that thorium too produced penetrating rays meant that this phenomenon
could no longer be "uranic rays". It was of a more general nature than
being a peculiarity of uranium.
2) All uranium compounds are active. The more uranium
they contain, the more active they are.
3) The careful quantitative results of M. Curie allowed
her to note that pitchblende (rich in uranium oxide) and chalcite( rich
in uranyl phosphate) had anomalously high activity, much more than uranium
itself.
"They are much more active
than uranium itself. This fact is very remarkable and leads one to believe
that these minerals contain an element which is much more active than uranium."
This observation established
a new technique for identifying elements, namely by noting their radioactive
properties.
The Curies then began to try
and isolate the active element from pitchblende. By the beginning of May
1898 they finally isolated a very active product which they thought was
pure enough to investigate spectroscopically. This sample showed no discernible
new spectral lines. They continued their chemical distillations of the
pitchblende products so that by June they had products 300 times more active
than uranium. By this time they had come to conclude that their were two
new active elements hiding in the pitchblende, one which accompanied bismuth
and another which accompanied barium in the breakdown from pitchblende.
They tried spectroscopy again,
with the aid of an expert, but again could not find spectroscopic evidence
of a new substance. Despite the lack of a spectroscopic signal they felt
their evidene based on the activity of the bismuth companion was sufficient
to give their new element a name, Po, polonium, after Marie's home country.
On July 18, H. Becquerel read
a paper contributed by the Curies ( neither were members of the Academy)
entitled "On a new radio-active substance
contained in pitchblende" to the Academy.
Note that this is the first introduction of the word "radioactivity" into
the scientific lexicon.
The next step of their research
that year was to try to find spectroscopic evidence for the other radio-active
element carried off with the barium. By October they had received another
shipment of pitchblende. By the end of November, with the help of Gustave
Bemont, a spectroscopist at the EPCI where Pierre taught, they had produce
a barium product which had an activity 900 times that of uranium. Spectroscopic
analysis of this sample by an eminent spectroscopist (Eugene Demarcay)
revealed a new spectral line. By the end of December 1898 they were able
to send off their next report to the Academy. It was read on December 26,
1898. A note was attached from Demarcay "This
ray ( i.e. spectral line) does not appear to me to be attributable to any
known element ... its presence confirms the existence, in small quantity,
of a new element in the barium chloride of M. and Mme Curie."
This element is Radium.
Marie Curie was determined to
isolate radium so as to be able to measure it's atomic weight and to prepare
compounds of it. Even the highly active samples they had isolated with
barium from the pitchblende did not contain enough radium that they could
measure a difference in mass between their sample and an ordinary sample
of barium chloride. Pierre was more interested in the phenomenon of radioactivity
itself. The task of separating out radium in weighable quantities became
Marie's quest.
Since radium was present in such
tiny amounts in pitchblende large quantities of pitchblende were needed.
Also, this work would entail considerable space. The Curies were given
a drafty hangar at EPCI. On rainy days they had to work inside the hangar
on the chemical treatments. Otherwise they did the processing outside.
With financial aid from Baron Edmond de Rothschild they obtained more than
ten tons of pitchblende from the Joachimsthal mine. This is residue left
over from uranium mining. By Spring 1899 Marie had everything she needed
to do the separation. Over the next few years Marie's highly active barium
samples were sought after by several physicsts and chemists. The Curies
were able to investigate the effects of magnetic fields on the emissions
from radium.
Finally in July 1902 Marie was
able to present a paper to be read at the Academy announcing that she had
succeeded in obtaining 0.1g of radium out of her mountain of pitchblende.
She concluded that its atomic weight was 225 (226 is the modern value)
and that it should appear in the periodic table after barium under the
column of alkaline earth metals.
It was very important to be able
to isolate radium and measure its chemical and atomic properties. Frederick
Soddy, in 1904, noted that all the other radioactive substances occurred
in such minute quantities that their only evidence of existence was their
radioactivity. In 1924 Jean Perrin stated "It
is not an exaggeration to say today that the isolation of radium is the
cornerstone on which the entire edifce of radioactivity rests."
PUBLIC FASCINATION WITH RADIUM
Radioactivity and radium were
fascinating subjects for the general public.
Quite soon it was realized that
tremendous energy was available in radioactive atoms. By 1905 everyone
who could read knew about radium.
From Susan Quinn's book, p 196:
"The usual tendency of the popular
press to exaggerate the practical benefits of scientific discovery was
carried to wild extremes in the case of radium. ... Charles H, Gage in
San Francisco, a man who 'has a laboratory and calls himself professor,'
claimed he could ' run a big automobile filled with passengers a distance
of 300 miles' on batteries charged with his 'new radium preparation'. "
It was soon recognized that radioactivity
could be useful in the treatment of cancer. However, quacks were quick
to sell radioactivity as a cure all, examples: ( from Susan Quinn's book
- p 410 )
- a radioactive "Curie Hair
Tonic" which stopped hair loss and returned it to its original color
- a "Creme Activa" which promised
eternal youth accompanied by a statement that " Madame Curie ... promises
miracles."
- from 1929, a list of eighty
patent medicines with radioactive ingredients which came in the form of
bath salts, liniment, suppositories, toothpaste, chocloate candies, etc.
However, reputable physicians
also used "radium therapy" for a range of illnesses besides cancer. Soldiers
in WWI were given intravenous injections of radium solutions in cases of
extreme blood loss, and external applications of radium and radon to soften
scar tissue, loosen joints and stimulate nerve function. Marie Curie herself
supplied sources for such treatment during the war.
http://musee.curie.fr/presentation/musee.html
Une "petite boutique du radium" présente différents
produits des années vingt reflétant l'engouement que
connaissait le radium à cette époque. Parmi ces
produits, une fontaine à eau radioactive, des publicités
vantant la laine pour bébé "la laine Oradium" ou la
crème de beauté "Tho-Radia" ! |
 |
Even as early as 1904 it was known
that high doses of ionizing radiation could be lethal. Pierre Curie's last
papers dealt with radiation effects on mice and guinea pigs. He himself
showed signs of radiation illness in the years before he died in a street
accident in 1906. Marie Curie died of pernicious anemia in 1934.
The Radium Institute
After the Curies were awarded
the Nobel prize in 1903, Pierre was offered a position as professor at
the Sorbonne. As a part of the contract hiring him the University agreed
to build a new laboratory to house the "Radium Institute" as well as an
adjoining institute for medical research.
Pierre's death in a street accident
in 1906 left his position unfilled. Marie Curie was offered is duties,
but not his position, at the Sorbonne. She directed the Radium Institute
until her death in 1934. Researchers at this institute discovered other
radioactive elements. It was also the site of the first production of artificial
radioactivity and almost the site where the neutron was discovered.